
A Decade of Growth & A Willingness to Experiment
The Challenge
The world was a very different place when Birnbach Communications and Lion Ridge started working with Seqens NA, a contract research organization (CRO) and contract manufacturing organization (CMO) back in 2011. Blogs, social media and SEO weren’t tools used by B2Bs. Thought leadership was different. The company, then known as PCI Synthesis, was different. A pharmaceutical manufacturer of new chemical entities (NCEs), active pharmaceutical ingredients (APIs), and other specialty chemical products, it was a 50-person operation.
A decade later and now a world-renowned Contract Development & Manufacturing Organization (CDMO), the company has tripled in size, and has a significant presence with key trade media and high visibility in the industry. And the success of our thought leadership program – combined with a coordinated SEO program executed by Lion Ridge Design – has been a factor in its growth and eventual acquisition by Seqens CDMO.
Strategic Execution At Work
Strategic Execution At WorkInitially, our program focused on media relations and announcements. But after a couple of years of success on behalf of the company, which included cover stories in trade magazines and local news media, we recommended making a change to thought leadership to provide a steady flow of content about key issues facing the company’s customers and the industry. The recommendation was based on several factors:
- The company didn’t always have a regular news flow, even though it was growing.
- Announcements tended to be contingent on the willingness of customers – known in the industry as sponsors – and sponsors weren’t always willing to talk because they considered the work PCI did as proprietary.
- We needed a way to address key issues facing sponsors, and announcements weren’t always effective because they didn’t address sponsors’ concerns.
- Content was fast becoming key and the fuel to effective social media programs and search engine optimization.
At the time, B2Bs in general, and CROs and CMOs in particular, did not do blogs or social media. But Ed Price, the company’s founder, was willing to experiment.
The Results over the past 5 years
Over the years, we’ve written hundreds of bylined articles that were published in industry leading publications and posts that appear on the company’s blog. These bylined articles (which led to a regular column in a key trade publication, and strategic blog posts address key issues facing sponsors, answer questions they have about working with an outsourcing organization, provide best practices and insights to help keep costs down and projects on time.
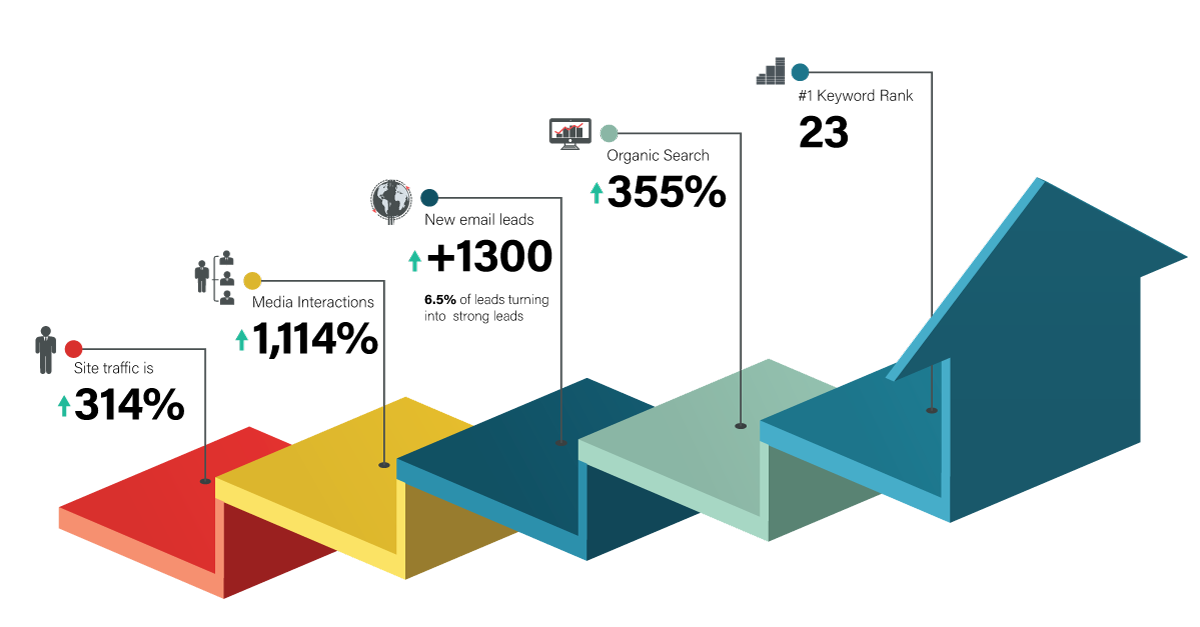
The blog and industry articles serve as the engine that drives traffic to the website, prompting emails and calls from potential sponsors interested in doing business with the company.
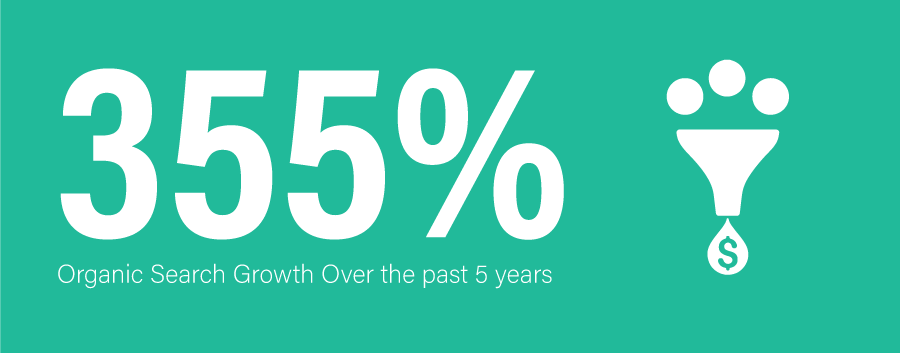
SEO awareness has increased exponentially through the program and when a search is conducted related to API manufacturing, Seqens is often the first name that pops up.
Our thought leadership campaign helped get the attention of Lyon, France-based Seqens CDMO, which in 2018 acquired PCI Synthesis. We expanded our work to provide PR and marketing to the parent company as well, which uses our blogs as part of a paid LinkedIn campaign, as we continue to work with Seqens North America.
Key coverage
More than 50 bylined articles published in Contract Pharma, Pharmaceutical Outsourcing, Pharma’s Almanac, Outsourced Pharma, Pharmaceutical Compliance Monitor, other U.S. and European trade publications.
1. Contract Pharma: “Trends Affecting the Generic Drugs Sector in 2020: Supply chain issues, the need for additional talented chemists, lower drug prices, growth of development in the U.S. will be key factors” (Feb. 21, 2020)
2. American Pharmaceutical Review: “The CDMO’s Challenges for Developing Oral Suspension for Children” (Jan./Feb. 2020)
3. Pharmaceutical Outsourcing: “The Industry Conundrum: Curbing Rising Drug Prices While Keeping Pharma Firms Profitable” (May 28, 2019)
4. Pharmaceutical Outsourcing: “What’s On the Minds of Biotech and Generic Drug Manufacturers in 2019?” (March 14, 2019)
5. Pharmaceutical Outsourcing: “How Do You Ensure a Smooth Technology Transfer in API Manufacturing? It’s All About Collaboration” (Aug. 9, 2018)
6. Chimica Oggi-Chemistry Today (Italy): “Panel Discussion on Custom Synthesis & Contract Manufacturing: The Chemistry between sponsors and CDMOs: The importance of Working Collaboratively before the project begins” (July/August 2018)
7. Pharmaceutical Outsourcing: “Top Mistakes in Analytical Method Validation and How to Avoid Them” (May 25, 2018)
8. Speciality Chemicals (U.K.): “Key trends for the generic drug development sector in 2018” (March/April 2018)
9. Pharmaceutical Outsourcing: “What Could Possibly Go Wrong in API Development?” (March 20, 2018)
10. Pharmaceutical Outsourcing: “What’s Shaping the New Year in Drug Development and Discovery?” (Feb. 10, 2018)